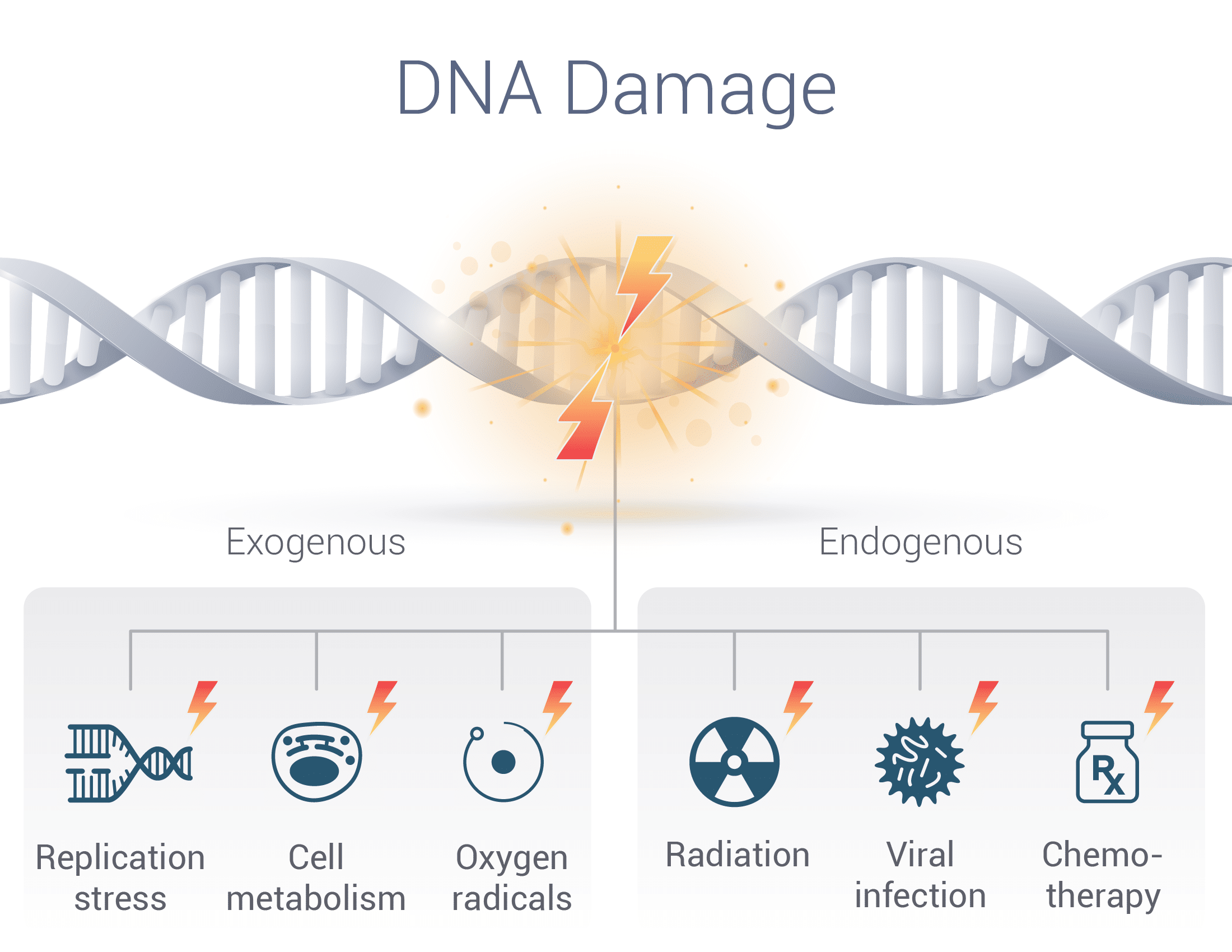
DNA Damage Overview Radiation may damage any of the components of DNA: Base damage Ultraviolet radiation (UV-A) typically causes base damage, such as 8-oxo-G and pyrimidine dimers (e.g. cyclobutane pyrimidine dimer, and 6-4 photoproduct), although can also lead to SSBs.
Full Answer
How can you repair your cells DNA damage?
Repair Your Cells DNA Damage With These Nutrients. Every hour of the day your cells are under attack. Normally, this is okay because your cells have a built-in DNA repair system that fixes any damage. But when your cells are undernourished, they can lose the ability to repair themselves.
Can we control radiation damage to our DNA?
But new research from Harvard Medical School just revealed exactly how that process can be controlled. See, when cells are exposed to radiation, a bad-guy protein known as DBC1 binds with PARP1, an innocent protein that would otherwise repair your messed-up DNA.
How does ionising radiation affect DNA replication?
DNA double-strand breaks constitute the most dangerous type of DNA damage induced by ionising radiation (IR). Accordingly, the resistance of cells to IR is modulated by three intimately related cellular processes: DNA repair, recombination, and replication.
How do DNA double-strand breaks induce ionising radiation?
DNA double-strand breaks constitute the most dangerous type of DNA damage induced by ionising radiation (IR). Accordingly, the resistance of cells to IR is modulated by three intimately related cellular processes: DNA repair, recombination, and replication. Significant discoveries in this field of research have been made over the last few years.
How long does it take to repair damaged DNA?
They found that the DNA of transcribed genes was just about fully mended in two circadian cycles, Sancar said. Restoration of these genes composed the majority of repair during the first 48 hours but afterward, repair of nontranscribed DNA became dominant and proceeded for weeks.
Can cells repair radiation damage?
Introduction. Radiation damage to cells can either occur directly or indirectly. [1] Most of the notable damage occurs to the cell DNA. Cells have repair mechanisms to fix this damage, but these mechanisms are not perfect and occassionally damage persists.
What repairs your DNA when it gets damaged?
Thus, enzymes known as DNA glycosylases remove damaged bases by literally cutting them out of the DNA strand through cleavage of the covalent bonds between the bases and the sugar-phosphate backbone. The resulting gap is then filled by a specialized repair polymerase and sealed by ligase.
Can radiation damage be reversed?
Damage by radiation is irreversible. Once the cells are damaged, they do not repair themselves. Until now, there is no way for medicine to do this, so it is important for someone who has been exposed to seek medical help as soon as possible.
Does DNA repair itself after radiation?
The radiation could damage the cell's DNA, but the DNA repairs itself. The radiation could prevent the DNA from replicating correctly. The radiation could damage the DNA so badly that the cell dies. This is called apoptosis.
Which radiation causes the most damage to DNA?
DNA double-strand breaks constitute the most dangerous type of DNA damage induced by ionising radiation (IR). Accordingly, the resistance of cells to IR is modulated by three intimately related cellular processes: DNA repair, recombination, and replication.
Is there a way to repair DNA?
Most damage to DNA is repaired by removal of the damaged bases followed by resynthesis of the excised region. Some lesions in DNA, however, can be repaired by direct reversal of the damage, which may be a more efficient way of dealing with specific types of DNA damage that occur frequently.
What are the 4 types of DNA repair?
At least five major DNA repair pathways—base excision repair (BER), nucleotide excision repair (NER), mismatch repair (MMR), homologous recombination (HR) and non-homologous end joining (NHEJ)—are active throughout different stages of the cell cycle, allowing the cells to repair the DNA damage.
How can you improve your DNA?
Replace with plant-based edibles such as vegetables, fruit and proteins like walnuts, beans and tofu. And we do believe that supplements like DHA, lutein, zeaxanthin, vitamin D-3, calcium and half a multivitamin twice a day are a good insurance policy against an imperfect diet.
What supplements help with radiation?
Some supplements, especially antioxidants, may change how radiation therapy works. Antioxidants include vitamins A, C, E, beta-carotene, and selenium, among others. Many people take antioxidants during treatment hoping they can protect normal tissues from treatment side effects.
How do you treat radiation damage?
If a person is exposed to a high amount of radiation, they will need to be decontaminated and may need transfusions of fluids, red blood cells, white blood cells, and platelets. If you have radiation side effects from cancer treatment, your doctor may be able to give you medications and ointments to reduce them.
How does radiation affect DNA?
Ionizing radiation directly affects DNA structure by inducing DNA breaks, particularly, DSBs. Secondary effects are the generation of reactive oxygen species (ROS) that oxidize proteins and lipids, and also induce several damages to DNA, like generation of abasic sites and single strand breaks (SSB).
How does radiation damage cells?
They have very high levels of chemical reactivity, and therefore generate indiscriminate chemical reactions. Radiation and electrons bombarded by radiation move haphazardly inside the cell, resulting in damage to the various molecules forming the cell. Chromosomal DNA inside the cell nucleus can also be damaged.
Does radiation damage cell walls?
Radiation can directly cause corrosive damage to the cell membrane or indirectly alter the biological characteristics of the cell membrane by affecting the composition of the cell membrane.
How does radiation damage the body?
Radiation can damage the DNA in our cells. High doses of radiation can cause Acute Radiation Syndrome (ARS) or Cutaneous Radiation Injuries (CRI). High doses of radiation could also lead to cancer later in life.
What is the most dangerous type of DNA damage induced by ionising radiation?
DNA double-strand breaks constitute the most dangerous type of DNA damage induced by ionising radiation (IR). Accordingly, the resistance of cells to IR is modulated by three intimately related cellular processes: DNA repair, recombination, and replication. Significant discoveries in this field of research have been made over the last few years.
What happens if a double helix impinges on DNA?
If such an event impinges on DNA, with the diameter of the double helix being approximately 2.5 nm, it would be expected to cause considerable local damage, including DSBs, single-strand breaks, and base damage.
How do cells respond to DSBs?
Cells respond to exogenous and endogenous DSBs through a cascade of proteins ranging from sensors, which recognise the damage, through signal and mediator proteins to a series of downstream effectors that induce cell-cycle arrests, complete repair by homologous or nonhomologous mechanisms, or alternatively trigger cell death by apoptosis (for a review, Abraham, 2001; Jackson, 2002 ). A simplified illustration is shown in Figure 1A. Defects at almost any step of this response pathway can result in measurable alterations of DNA repair by HR and/or NHEJ. Mutations upstream in the cascade, before the decision is made whether a lesion is repaired by HR or NHEJ, can directly affect both principal recombinational repair pathways. For example, mutation of the upstream kinase ATM, which is mutated in ataxia telangiectasia (AT), impairs HR and NHEJ ( Luo et al, 1996; Khanna and Jackson, 2001 ), although it is yet unclear whether these alterations underlie the pronounced radiation hypersensitivity of AT cells. Loss of the Mre11 protein, which is mutant in the AT-like disorder (ATLD) and is usually found in a complex with Nbs1 and Rad50 (MRN complex), reduces both HR and NHEJ, which likely contributes to radiation hypersensitivity ( Zhang et al, 2004; Zhang et al, unpublished). The MRN complex may be involved in the processing of DNA ends, among other functions, prior to the repair by HR or NHEJ. Both of the tumour suppressors p53 and BRCA1 control aspects of HR and NHEJ ( Willers et al, 2000; Akyuz et al, 2002; Zhang et al, 2004; Willers et al, unpublished; Dahm-Daphi et al, unpublished), but the impact of these regulations on radiation resistance is a subject of active study. FANC-D2, which is mutated in a small subset of patients with the rare cancer predisposition and chromosomal instability syndrome Fanconi anaemia (FA), is likely implicated in HR via its protein interaction with BRCA2 (i.e., FANC-D1) ( Howlett et al, 2002) and confers cellular radiation resistance – in contrast to most of the other genes in the FA pathway. Similarly, mutations of genes involved in execution of HR, such as BRCA2 or Rad51, compromise HR and radiation resistance, while mutations in the pathway controlled by DNA-PK affect radiation resistance via disruption of NHEJ. Increasing evidence points towards the existence of multiple subpathways of HR and NHEJ ( Tutt et al, 2001; Akyuz et al, 2002; Jackson, 2002; Zhang et al, 2004 ), which will further complicate the understanding of the determinants of radiation resistance.
What are the consequences of a mutation in the BRCA1–BRCA2–Rad51 pathway?
As we already discussed, mutations in the BRCA1–BRCA2–Rad51 pathway are associated with defective HR, and these may not only result in genomic instability but also determine the resistance of tumour cells to exogenous DSBs or ICLs. Conversely, inappropriate upregulation of HR activities could also contribute to genomic instability by causing homology-mediated aberrations such as certain chromosomal translocations or loss of heterozygosity. Some of these observations may be linked either to the observed overexpression of the Rad51 protein in several cell types or to the widespread disruption of pathways controlled by the p53 gene ( Maacke et al, 2000; Linke et al, 2003 ), but it is yet unclear how these alterations affect radiation resistance (in the absence of apoptosis) ( Albrechtsen et al, 1999; Dahm-Daphi, 2000; Böhnke et al, 2004 ). A direct clinical application of impaired HR control was suggested recently by the finding that the activity of the FA pathway, as determined by the methylation status of the FANC-F gene, dictated the sensitivity of several ovarian tumours to cisplatin ( Taniguchi et al, 2003 ). Disruption of the pathway was found in 8–21% of tumours. An attractive hypothesis to be tested in clinical studies will be whether also methylation of BRCA1 in a significant fraction of sporadic breast and ovarian cancers, and possibly of FANC-F in breast tumours, can determine the response of such cancers to treatment with cross-linking drugs. Whether it will be possible to use the functional status of the FA pathway to predict the clinical tumour response to IR seems to be less clear.
What are the three R's in DNA replication?
Here, we have focused on some of the concepts and principles in the study of DNA replication, recombination, and repair (the three ‘R's’). These processes, which can no longer be considered separately, form a new paradigm for the understanding of the cellular resistance to radiation treatment. Genetic mutations in recombinational processes that affect replication and DSB repair may not only promote genomic instability but also determine the response of tumours to combination therapies with DNA damaging agents. This concept may provide the framework for future pre-clinical and clinical studies that discover and test novel combination therapies and tailor these to individual tumours. While some of our considerations are speculative at the present time, we anticipate that the rapid progress in this exciting field of research will continue over the next few years and provide many of the answers. Finally, although the focus of this review has been the contribution of cellular radiation resistance to the clinical tumour response, it is clear that additional factors, such as the contribution of the tumour microenvironment, are at least equally important in determining the likelihood of achieving tumour control and cure.
What is a double strand break?
It is established that the creation of a DNA double-strand break (DSB) represents the principal lesion that, if not adequately repaired, can lead to cell death via the generation of lethal chromosomal aberrations or the direct induction of apoptosis.
Is NHEJ a DSB repair pathway?
Still, NHEJ is suggested to be the dominant DSB repair pathway in mammalian cells, which is in part related to the fact that only a very small fraction of the genome is coding for genes and regulatory elements, and that small sequence changes are tolerated by the cells.
What is the process of repair of DNA?
Repairing damage in DNA from anything that causes a mutation, such as UV radiation and tobacco smoke, is a fundamental process that protects our cells from becoming cancerous.
Why do DNA sequences increase the number of mutations?
The researchers showed that the numbers of DNA mutations are increased in gene promoters because the proteins that bind DNA to control gene expression block one of our cell repair systems responsible for fixing damaged DNA. ...
Which gene is responsible for cancer?
Internationally, scientists have so far identified only one promoter mutation, known as the telomerase reverse transcriptase (TERT) gene , that definitively contributes to cancer. "Our study highlights the need for further research on the role of gene promoter mutations in cancer development," Dr Wong said.
Does DNA replication show the same competence?
Nov. 7, 2017 — Error surveillance and repair mechanisms during DNA replication do not show the same competence in all regions of the human genome. Scientists have discovered that the mechanism that repairs errors ...
Does UV light damage DNA?
May 30, 2019 — UV light damages the DNA of skin cells, which can lead to cancer. This process is counteracted by the DNA repair machinery. It has been unclear, however, how repair proteins work on DNA tightly ...
Can DNA repair damage mice?
Feb. 12, 2019 — Researchers have discovered how overactive DNA repair systems can lead to retinal damage and blindness in mice. A DNA repair enzyme called Aag glycosylase becomes hyperactive, provoking an ...
Why is it okay to repair DNA damage?
Every hour of the day your cells are under attack. Normally, this is okay because your cells have a built-in DNA repair system that fixes any damage. But when your cells are undernourished, they can lose the ability to repair themselves. And that’s bad news.
Can zinc interfere with DNA?
Zinc can interfere with the absorption of these minerals. To avoid any of these problems, I simply take my zinc supplement before I go to bed. Store it in a dark place at room temperature. To Your DNA’s Good Health, Be Sure to read more in the next post about an exciting nutrients that helps repair DNA damage as well.
Does zinc help with cell damage?
An Aug. 2009 study by the Linus Pauling Institute at Oregon State University shows that supplementing with zinc reverses cell damage. Add this latest finding to zinc’s list of health benefits: – Heart-health booster. – Essential to your prostate and sexual performance. – Can prevent pneumonia and speed the recovery from colds.
